The information on this website is for healthcare professionals. The COMET system is only offered to European hospitals or researchers with applicable approvals.
Our organ's cells need oxygen to function. Organs fail when cells lack oxygen.
Do the cells have enough oxygen? Are interventions necessary? How effective are they? In many situations only signs of damage allow physicians to answer these questions with confidence - when it is too late.
Now there finally is a way to measure oxygen inside active cells. This is quite different from current measurements in blood. Only in the cells themselves can it be determined if there is enough oxygen or if cells are hypoxic - and also if cells can't use the oxygen they receive.
COMET MEASUREMENT SYSTEM
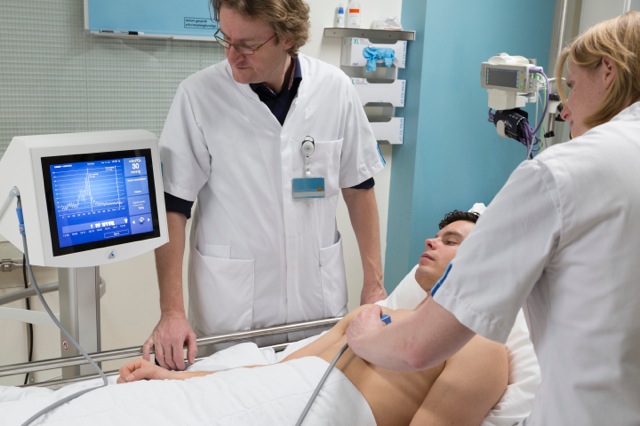
The CE-marked COMET measurement system determines oxygen availability as partial pressure of oxygen [mmHg]. It non-invasively measures oxygen in human skin cells with a high concentration of protoporphyrin IX - in real time at the bedside and only in the mitochondria of active cells.
We expect that the measurement of oxygen in the cells will enable doctors to guide the many interventions to ensure oxygen delivery to cells and that this will make care easier, safer and cheaper. Skin is regarded as an early warning sensor, so should give physicians time to prevent lasting damage. Preclinically, transfusion need was detected two hours before kidney failure. Monitoring cellular oxygen in skin should therefore provide early warning of complications and allow doctors to assess the necessity for interventions and follow their effects in real time. It should also facilitate decisions before and during peripheral interventions and be useful to detect alterations of cellular metabolism and help develop treatments for metabolic dysfunction. Measurements have also been performed in the gut of volunteers and preclinically in other organs. So far, a CE marked sensor is only available for the skin. Eventually, images of cellular oxygen distributions in organs should help assess tissue function for diagnosis, interventions or wound care.
Use the COMET measurement system as a low-burden adjunct to your studies and improve patient safety. Contact us for a quote (Europe only).
THE SCIENCE OF CELLULAR MONITORING
Anesthesiologist Dr. Egbert Mik, building on his invention made together with Dr. Michiel Sinaasappel, developed the protoporphyrin IX triplet state lifetime technique (PpIX-TSLT). It is the first practical way to clinically measure oxygen availability and consumption where oxygen is needed: in the tissue cells rather than in the blood. It uses the oxygen dependent duration of the 'glow-in-the-dark' of the precursor to heme. This protoporphyrin IX is metabolized in epidermal mitochondria after local priming with aminolevulinic acid used in photodynamic therapy or diagnosis. The signal therefore originates only in active mitochondria and reflects the balance between oxygen supply and demand. The COMET's skin sensor collects a signal from several square millimeters of epidermis, providing an average value for the different cells. In a preclinical trial the oxygenation of cells in the gut has been measured with an endoscopic sensor. Ultimately, interstitial probes, even imaging, should be feasible with this technology. Oxygen should be measured where it is needed so doctors can treat what matters.
Editors of Anesthesiology summarized a publication of research results with a prototype of the COMET and wrote that directly measuring tissue oxygenation "would be a major advance for perioperative medicine." O'Brien and Schmidt wrote in a separate editorial for Anesthesiology (July 2016): "A reliable measure of oxygen tension at the level of the mitochondria might significantly refine transfusion practice... Indeed, any clinical scenario where cellular oxygenation might be compromised could potentially benefit... If mitochondrial PO2 can be measured reliably in humans, the potential value of this technique is hard to overestimate."
A range of cellular oxygen availability is expected for different cells depending on their positions along capillaries and their distances from them (the Krogh model and its refinements e.g., here or here, provide theoretical explanations). Intense perfusion can result in average epidermal mitoPO2 values only slightly below the arterial oxygen tension. Temporary local pressure stops microvascular blood flow in the measurement region. Repeated measurements during and after pressure allow determination of cellular oxygen utilization and analysis of re-perfusion.
Results with the technology and its application have been published in or presented at:
- Hilderink BN et al (2025). Low postoperative mitochondrial oxygen tension is an early marker of acute kidney injury after cardiac surgery: A prospective observational study. J Crit Care 88 155088. https://doi.org/10.1016/j.jcrc.2025.155088
-
Flick M et al. (2025) Personalized intraoperative arterial pressure management and mitochondrial oxygen tension in patients having major non‑cardiac surgery: a pilot substudy of the IMPROVE trial. Journal of Clinical Monitoring and Computing https://doi.org/10.1007/s10877-024-01260-0
-
De Wijs C et al. (2024) Mitochondrial oxygenation monitoring and acute kidney injury risk in cardiac surgery: A prospective cohort study. Journal of Clinical Anesthesia 101 111715 https://doi.org/10.1016/j.jclinane.2024.111715
-
Yang Y et al. (2024) COMET: monitoring mitochondrial shock in anesthesiology and intensive care medicine. Anesthesiology and Perioperative Science 2:41 https://doi.org/10.1007/s44254-024-00079-x
-
Hilderink BN et al. (2024) Rapid non-invasive measurement of mitochondrial oxygen tension after microneedle pre-treatment: a feasibility study in human volunteers. Journal of Clinical Monitoring and Computing https://doi.org/10.1007/s10877-024-01249-9
-
Hilderink BN et al. (2024) Hyperoxemia and hypoxemia impair cellular oxygenation: a study in healthy volunteers. Intensive Care Medicine Experimental 12:37 https://doi.org/10.1186/s40635-024-00619-6
-
Baysan M et al. (2024) Description of mitochondrial oxygen tension and its variability in healthy volunteers. PLoS ONE 19(6): e0300602. https://doi.org/10.1371/journal.pone.0300602
-
Ubbink R et al. (2023) Measuring Mitochondrial Oxygen Tension during Red Blood Cell Transfusion in Chronic Anemia Patients: A Pilot Study. Biomedicines 2023, 11, 1873. https://doi.org/10.3390/biomedicines11071873
-
Hilderink BN et al. (2023) A simulation of skin mitochondrial Po2 in circulatory shock. J Appl Physiol 134:1165-1176, 2023. https://journals.physiology.org/doi/full/10.1152/japplphysiol.00621.2022
-
Harms FA et al. (2023) Monitoring of mitochondrial oxygen tension in the operating theatre: An observational study with the novel COMET® monitor. PLoS ONE 18(2):e0278561. https://doi.org/10.1371/journal.pone.0278561
-
Ubbink R. (2022) Clinical Application of Mitochondrial Oxygen Tension Measurement. PhD Thesis Rotterdam 2022. https://pure.eur.nl/en/publications/clinical-application-of-mitochondrial-oxygen-tension-measurement
-
Neu C et al. (2022) Body composition, mitochondrial oxygen metabolism and metabolome of patients with obesity before and after bariatric surgery (COMMITMENT): protocol for a monocentric prospective cohort study. BMJ Open 2022;12:e062592. doi:10.1136/bmjopen-2022-062592
-
HarmsFA et al, (2022) Mitochondrial Oxygenation During Cardiopulmonary Bypass: A Pilot Study. Front.Med.9:785734. doi:10.3389/fmed.2022.785734
-
Ubbink R. (2021) Quantitative intracellular oxygen availability before and after 5-aminolevulinic acid skin photodynamic therapy. Photodiagnosis and Photodynamic Therapy 36 (2021) 102599 https://doi.org/10.1016/j.pdpdt.2021.102599
-
Mandigers L et al. (2021) Monitoring Mitochondrial Partial Oxygen Pressure During Cardiac Arrest and Extracorporeal Cardiopulmonary Resuscitation. An Experimental Pilot Study in a Pig Model. Front. Cardiovasc. Med. 8:754852. doi: 10.3389/fcvm.2021.754852
-
Harms FA et al. (2021) Monitoring of mitochondrial oxygenation during perioperative blood loss BMJ Case Rep 2021;14:e237789. doi:10.1136/bcr-2020-237789
-
Wefers Bettink M. (2021) Mind the Mitochondria. PhD Thesis Rotterdam 2021. https://pure.eur.nl/en/publications/mind-the-mitochondria
-
Ubbink R et al. (2020) Mitochondrial oxygen monitoring with COMET: verification of calibration in man and comparison with vascular occlusion tests in healthy volunteers. Journal of Clinical Monitoring and Computing 2020 https://doi.org/10.1007/s10877-020-00602-y
-
Neu C et al. (2020) Non-invasive Assessment of Mitochondrial Oxygen Metabolism in the Critically Ill Patient Using the Protoporphyrin IX-Triplet State Lifetime Technique—A Feasibility Study. Front. Immunol. 11:757. doi: 10.3389/fimmu.2020.00757
-
Mik EG et al. (2020) Monitoring mitochondrial PO2: the next step. Curr Opin Crit Care 2020, 26:289–295. DOI:10.1097/MCC.0000000000000719 https://journals.lww.com/co-criticalcare/Abstract/2020/06000/Monitoring_mitochondrial_PO2__the_next_step.11.aspx
-
Costerus SA. (2020) Mitochondrial Oxygen Monitoring During Surgical Repair of Congenital Diaphragmatic Hernia or Esophageal Atresia: A Feasibility Study. Front.Pediatr.8:532. doi:10.3389/fped.2020.00532 https://www.frontiersin.org/journals/pediatrics/articles/10.3389/fped.2020.00532/full
-
Baysan M et al. (2020) Study protocol and pilot results of an observational cohort study evaluating effect of red blood cell transfusion on oxygenation and mitochondrial oxygen tension in critically ill patients with anaemia: the INsufficient Oxygenation in the Intensive Care Unit (INOX ICU-2) study. BMJ Open 2020;0:e036351. doi:10.1136/bmjopen-2019-036351 https://bmjopen.bmj.com/content/10/5/e036351
-
Baumbach P et al. (2020) A Pilot Study on the Association of Mitochondrial Oxygen Metabolism and Gas Exchange During Cardiopulmonary Exercise Testing: Is There a Mitochondrial Threshold? Front. Med. 7:585462. doi: 10.3389/fmed.2020.585462 https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2020.585462/full
-
Wefers Bettink M et al. (2019) Review Article. Mind the mitochondria! J Emerg Crit Care Med 2019;3:45 http://dx.doi.org/10.21037/jeccm.20 http://dx.doi.org/10.21037/jeccm.2019.08.08
- v Dijk LJD et al. Oxygen-dependent delayed fluorescence of protoporphyrin IX measured in the stomach and duodenum during upper gastrointestinal endoscopy. J Biophotonics. 2019 May 29:e201900025. doi: 10.1002/jbio.201900025
- Baumbach P, Neu C et al. A pilot study of exercise-induced changes in mitochondrial oxygen metabolism measured by a cellular oxygen metabolism monitor (PICOMET). BBA - Molecular Basis of Disease 1865 (2019) 749–758
- Wefers Bettink M et al. Monitoring of mitochondrial oxygen tension in the operating theatre: first experiences with the novel COMET monitor. Presentation 1198 at ESICM Lives 2018 in Paris.
- Ubbink R et al. Monitoring of mitochondrial oxygen tension during a red blood cell transfusion and fluid challenge in chronic anemia patients. Presentation 970 at ESICM Lives 2018 in Paris.
- Ubbink et al. Probing Tissue Oxygenation by Delayed Fluorescence of Protoporphyrin IX. Chapter 13 in Dmitri B. Papkovsky and Ruslan I. Dmitriev. Quenched-phosphorescence Detection of Molecular Oxygen: Applications in Life Sciences. The Royal Society of Chemistry 2018 ISSN: 2052-3068 DOI: 10.1039/9781788013451
- Wefers Bettink M et al. Non-invasive measurement of mitochondrial oxygenation and respiration in human endotoxemia and sepsis. Session 556 at IARS Annual Meeting Chicago 29 April 2018.
- v Diemen MPJ et al. Measurement of Oxygen Metabolism In Vivo. p.315-322. Chapter 20 of Will Y, Dykens J.A. (Editors). Mitochondrial Dysfunction Caused by Drugs and Environmental Toxicants. Wiley 2018. ISBN 978-1-119-32974-9
- Mik EG. Better assessment of cellular oxygenation. The Future of monitoring. Update on Monitoring in the Acutely Ill Patient. Rome, Dec 11 2017
- Mik EG. New method to evaluate cellular metabolism. Update on monitoring. Update on Monitoring in the Acutely Ill Patient. Rome, Dec 11 2017
- v Diemen MPJ et al. Validation of a pharmacological model for mitochondrial dysfunction in healthy subjects using simvastatin: A randomized placebo-controlled proof- of-pharmacology study. Europ. J. Pharmaco. 815 (2017);290-297
- Ubbink R et al. Novel technique to monitor effect of transfusion on mitochondrial oxygenation. Poster P251 presented at 37th ISICEM 21 March 2017
- Mikhael M, Nasr N, Al Jindi P. Cutaneous Mitochondrial PO2 : A Beginning of a New Era? Anesthesiology Vol.126, p. 348
Römers et al. In Reply. Anesthesiology Vol.126, pp. 349-350 - Ubbink R et al. A monitor for Cellular Oxygen METabolism (COMET): monitoring tissue oxygenation at the mitochondrial level. J Clin Monit Comput (2017) 31: 1143. https://doi.org/10.1007/s10877-016-9966-x
- Nederlof R et al. Reducing mitochondrial bound hexokinase II mediates transition from non-injurious into injurious ischemia/reperfusion of the intact heart. J Physiol Biochem. 2016; 73(3): 323–333.
- Huntosova V. Effect of PpIX photoproducts formation on pO2 measurement by time-resolved delayed fluorescence spectroscopy of PpIX in solution and in vivo. J Photochem Photobiol B. 2016 Nov;164:49-56
- Zwaag J, Wefers Bettink MA, Kox M, Mik EG. Change of mitochondrial function in vivo during human endotoxemia: preliminary data. Nederlands tijdschrift voor anesthesiologie 27:100 (2016)
- Harms FA et al. Cutaneous Respirometry as Novel Technique to Monitor Mitochondrial Function: A Feasibility Study in Healthy Volunteers. PLoS One. 2016 Jul 25;11(7):e0159544 (2016)
- O’Brien EO, Schmidt U. Cellular Hypoxia in a Brand New Light (Editorial). Anesthesiology 2016 Jul; 125(1): 20-1 (2016)
- Romers LH et al. Cutaneous Mitochondrial PO2, but not Tissue Oxygen Saturation, Is an Early Indicator of the Physiologic Limit of Hemodilution in the Pig. Anesthesiology 2016 Jul;125(1):124-32 (2016)
- Ince C et al. Microcirculatory and mitochondrial hypoxia in sepsis, shock, and resuscitation. J Appl Physiol 120(2):226-35 (2016)
- Freebody M. Delivering optical devices to the medical market. Photonics Spectra 40(4):50-54 (2016)
- Harms FA et al. Non-invasive monitoring of mitochondrial oxygenation and respiration in critical illness using a novel technique. Crit Care.19(1):343 (2015)
- Harms FA et al. In vivo assessment of mitochondrial oxygen consumption. In: Weissig, Edeas (eds.). Mitochondrial Medicine: Vol. 1, Probing Mitochondrial Function. Methods Mol Biol. 2015;1264:219-29.
- Harms FA et al. Cutaneous mitochondrial respirometry: non-invasive monitoring of mitochondrial function. J Clin Monit Comput. 2015 Aug;29(4):509-19 (2014)
- Harms FA. Towards non‐invasive monitoring of mitochondrial function. PhD Thesis ErasmusMC University Medical Center Rotterdam (2014)
- Mik EG et.al. Towards real-time measurement of intracellular oxygen tension Photonic International/2013 pp.52-53 (2013)
- Mik EG Measuring Mitochondrial Oxygen Tension: From Basic Principles to Application in Humans. Anesthesia & Analgesia 117(4) p 834-845 (2013)
- Harms FA et al. Cutaneous respirometry by dynamic measurement of mitochondrial oxygen tension for monitoring mitochondrial function in vivo. Mitochondrion S1567-7249(12)00226-7 (2012)
- Piffaretti F et al. Real-time, in vivo measurement of tissular pO2 through the delayed fluorescence of endogenous protoporphyrin IX during photodynamic therapy. J. Biomed. Opt. 17(11) 115007 (2012)
- Harms FA et al. Validation of the protoporphyrin IX-triplet state lifetime technique for mitochondrial oxygen measurements in the skin. Optics Letters 37: 2625-2627 (2012)
- Bodmer SI et al.. Microvascular and mitochondrial PO2 simultaneously measured by oxygen-dependent delayed luminescence. J.Biophotonics 5(2):140-151 (2012)
- Harms FA et al. Oxygen-dependent delayed fluorescence measured in skin after topical application of 5-aminolevulinic acid. J.Biophotonics 4(10): 731-9 (2011)
- Piffaretti et al. Optical fiber-based setup for in vivo measurement of the delayed fluorescence lifetime of oxygen sensors. J. Biomed. Opt. 16:037005 (2011)
- Mik EG Measuring Microvascular and Mitochondrial Oxygen Tension – Novel Techniques for Studying Tissue Oxygenation, PhD Thesis, University of Amsterdam (2011)
- Mik EG et al. Mitochondrial oxygen tension within the heart. J. Moll. Cell. Cardiol. 46: 943-951 (2009)
- Mik EG et al.. In vivo mitochondrial oxygen tension measured by a delayed fluorescence lifetime technique. Biophys. J. 95: 3977-3990 (2008)
- Mik EG et al. Mitochondrial PO2 measured by delayed fluorescence of endogenous protoporphyrin IX. Nature Methods 3: 939-945 (2006)
